


|
Approximate Energy conversion factors: |
|
Approximate Energy conversion factors Table:
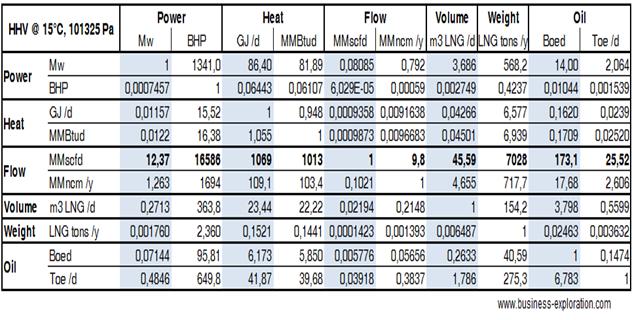
Selling to the Oil & Gas and Energy industry, requires to jump from natural gas to oil, from power to physical quantities: tons, barrels, cubic meters, from British to international standards…This table wants to be a hand while gauging a new or an old project, a prospective investment or the purchasing of a new equipment.
These factors are taken into account: Oil barrels, BOED, Tons of Oil, TOE / day LNG natural gas cubic meters, metric tons per day Methane normal cubic meters Ncm, standard cubic feet scf, per day or per year Heat equivalent Giga Joules, GJ, or British Thermal Units BTU Power in Mega watts, MW or British Horse Power BHP
Note the 6 categories: Power, Heat, Flow, Volume, Weight and Oil. They should guide you.
Assumptions behind these figures: The first is that we choose absolute value. For Natural Gas, e.g., we choose an HHV of 55.5 Mj/Kg. This is theoretical. No pipeline will give you gas with such a purity. But then you can apply your corrective factors, your efficiency factors, and come to a conclusion. The second is that we did our bet, both in researching accurate values, and doing accurate calculations. But you use this table at your own risk. However, if you discover an error, we will be happy to know and thank you in advance. The third is the basic figures I used. They are listed in the second table at bottom page.
+ Want to challenge old business models and inject new ideas? Get my "Little nightly thoughts" ! Join my mailing list, here.
|


|
Despite the openness of the cooperation, we are fully aware of the importance of confidentiality. We deal everyday with Hot Topics of CEOs and Top Managers of our Client’s Companies. We help them on long term and short term strategies to beat competition, create more profits, realign organizations, choose the best profiles for their team, assess M&A and Joint Ventures. This is the reason why you do not find here a full list of Clients and we do not quote the Author of the comments we reproduce. We can not risk to share any hint to their competitors. We do not share information if not authorized in writing, we jealously keep all records under secure systems, and do not make names or show data to any one, that can cause damage to our Clients and Partners, in any form: breaching copyright and intellectual property, offering insider trading hints, loosing sensitive, personal or strategic information in favor of competition etc. We do not share email addresses of our contacts with anyone and use them only to keep our network informed about our latest developments. Flavio Tosi, Principal - Business Exploration |

|
Privacy policy: |


|
VDR free tool |

|
Gas properties for Natural Gas components |

|
Unit of Measure |
Value |
note |
|
ncf/nmc |
35,31 |
|
|
scf/nmc |
37,33 |
|
|
cal/J |
4,1868 |
|
|
j/btu |
1055,056 |
|
|
t/b |
7,3 |
API 33 |
|
BHP/Kw |
0,7457 |
|
|
CH4 Mol weight |
16,043 |
g/mol |
|
R |
8,31434 |
j/mol/K |
|
CH4 HHV |
55,500 |
@ 15,4 °C MJ/kg |
|
CH4 LHV |
55,009 |
@ 15,4 °C MJ/kg |
|
molar volume CH4 (cm/mol) |
0,022354315 |
@ 0 °C , 101325 Pa |
|
molar volume CH4 (cm/mol) |
0,023593844 |
@ 15 °C , 101325 Pa |
|
density kg/ncm |
0,71766905 |
@ 0 °C , 101325 Pa |
|
density kg/scm |
0,6799655 |
@ 15 °C , 101325 Pa |
|
liquid density kg/cm |
422,36 |
@ 101325 Pa |
|
mol/nmc |
44,73409275 |
|
|
mol/scm |
42,38393692 |
|
|
scm/ncm |
1,05544921 |
measured |
|
scm/ncm |
1,057714421 |
pv=nrt |
|
TOE |
41,868 |
Gj |
|
1 barrel |
158,9873 |
liters |
|
oil density |
0,858 |
kg/liter @ 59 °F |
|
oil density |
7,335 |
barrels/metric ton |
|
1 boe |
5,8 * 10 ^6 |
Btu @ 59 °F - HHV |
|
oil HHV |
45,25 |
Mj/kg @59 °F |
|
oil HHV |
38,84 |
Mj/liter |
|
oil LHV |
42,98 |
Mj/kg @59 °F |
|
oil LHV |
36,89 |
Mj/liter |
|
www.business-exploration.com |
||
|
Component |
Mole Wt |
Hydrogen Atoms |
Carbon Atoms |
Cp (1) |
HHV (2) |
LHV |
Auto-ignition T (F) (3) |
Flame Speed @ xx (in/s)(4) |
||||
|
0.60 |
0.80 |
1.00 |
1.20 |
1.40 |
||||||||
|
H2 |
2.0159 |
2 |
0 |
3.4010 |
324.2 |
273.9 |
752 |
31.74 |
56.10 |
78.99 |
93.75 |
106.30 |
|
C1 |
16.0430 |
4 |
1 |
0.5266 |
1009.7 |
909.1 |
999 |
3.83 |
10.95 |
14.65 |
12.54 |
5.67 |
|
C2 |
30.0690 |
6 |
2 |
0.4080 |
1768.7 |
1617.8 |
959 |
4.49 |
11.12 |
15.91 |
16.32 |
10.40 |
|
C3 |
44.0960 |
8 |
3 |
.03887 |
2517.2 |
2315.9 |
871 |
5.36 |
11.48 |
15.53 |
15.42 |
9.51 |
|
IC4 |
58.1220 |
10 |
4 |
0.3867 |
3256.6 |
3001.0 |
864 |
6.34 |
12.58 |
17.39 |
18.04 |
13.67 |
|
NC4 |
58.1220 |
10 |
4 |
0.3951 |
3262.0 |
3010.5 |
761 |
5.03 |
10.50 |
14.44 |
13.89 |
7.87 |
|
IC5 |
72.1510 |
12 |
5 |
0.3829 |
3999.7 |
3697.9 |
788 |
From CompressorTech2 |
||||
|
NC5 |
72.1510 |
12 |
5 |
0.3880 |
4008.7 |
3706.8 |
496 |
|||||
|
C6 |
86.1780 |
14 |
6 |
0.3857 |
4756.1 |
4403.9 |
433 |
|||||
|
C7 |
100.2050 |
16 |
7 |
0.3842 |
5502.8 |
5100.3 |
433 |
|||||
|
C8 |
114.2320 |
18 |
8 |
0.3831 |
6248.9 |
5796.1 |
428 |
|||||
|
H2S |
34.0760 |
2 |
0 |
0.2370 |
586.7 |
637.0 |
500 |
|||||
|
(1) Specific heat Cp at costant pressure conditions near atmospheric |
||||||||||||
|
(2) Heating values in BTU/scf at 14.696 psia, 60°F, and uncorrected for compressibility from GPA 2545-09 |
||||||||||||
|
(3) Auto-ignitions temperatures from “Flammability Characteristics of Combustible Gases & Vapors” |
||||||||||||
|
(4) Laminar Flame Speed from University of Southern California Combustion Kinetics Laboratory |
||||||||||||
|
C1 methane |
||||||||||||
|
C2 ethane |
||||||||||||
|
C3propane |
||||||||||||
|
C4 isobutane - normal Butane |
||||||||||||
|
C5 isopentane |
||||||||||||
|
C6 hexane |
||||||||||||
|
C7 heptane |
||||||||||||
|
C8 octane |
||||||||||||
|
H2S hydrogen sulfide |
||||||||||||
|
H2 hydrogen
|
||||||||||||